This protocol is judged by an institutional evaluation board, an unbiased team that critiques any medical trials involving human beings. If a study requires a Program I drug, once the board approves the protocol as ethical, the researchers should apply for an investigational new drug (IND) selection from the FDA.Usage of unlawful medications is �
 Joshua Jackson Then & Now!
Joshua Jackson Then & Now! Mike Vitar Then & Now!
Mike Vitar Then & Now!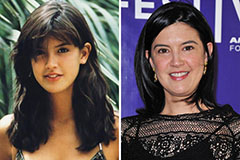 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now! Dawn Wells Then & Now!
Dawn Wells Then & Now!